We are delighted to announce the first people are enrolled in the UK’s Early Access to Medicines Scheme (EAMS) for Raxone.
Janet Bloor, Chair of Trustees of Action Duchenne said, “At Action Duchenne we were encouraged by the positive EAMS opinion, earlier this summer. Particularly, for young people living with Duchenne who have respiratory decline. I am delighted that respiratory function is being considered by the regulatory agencies. This will hopefully pave the way for more potential treatments that may benefit the wider spectrum of Duchenne patients.”
In June, Raxone (idebenone) was the first drug to be approved under the EAMS scheme for people living with Duchenne with respiratory function decline not taking glucocorticoids.
What Raxone being given approval through the EAMS scheme means to the Duchenne community
The decision by the MHRA allows people living with Duchenne, who meet the criteria defined under this scheme, to gain access to Raxone.
“I am pleased to be able to offer Raxone to several of my patients in respiratory decline, as there are no other medical treatments available,” said Dr. Dipansu Ghosh, a respiratory physician at a Duchenne centre based in Leeds.
To date, 15 specialist Duchenne centres in the UK have received training under the requirements of the EAMS. Several additional sites have expressed an interest to be trained and are currently undergoing local approval processes.
What is EAMS?
The UK’s industry-sponsored EAMS aims to give patients with life threatening or seriously debilitating conditions access to medicines that do not yet have a marketing authorization when there is a clear unmet medical need. Under the scheme, the MHRA provides a scientific opinion on the benefit/risk balance of the medicine, based on the data available when the EAMS submission was made. The opinion lasts for a year and can be renewed. The scheme is voluntary and the opinion from MHRA does not replace the normal licensing procedures for medicines.
About Raxone
Raxone is a 150mg tablet that contains a substance called idebenone and is to be taken with food and swallowed with a glass of water.
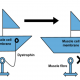
What does the treatment aim to do?
The treatment is designed to slow the progressive rate of decline in breathing ability for those with Duchnene. The drug works by aiming to help slow down the weakening of muscles in respiration and through this slow down the emergence of serious breathing difficulties for those living with Duchenne.
Who is eligible to take Raxone?
Those living with Duchenne who are 10 years old and over who are not taking glucocorticoids (steroids) and through a series of tests are shown to be suffering from a decline of respiratory function. This includes those who have previously been treated with steroids and those who cannot tolerate steroids.
It is important to note that Raxone is not a replacement for steroids and we would advise against those living with Duchenne stopping taking steroids for the sole reasons of being able to take Raxone.
All the details for patients who are eligible to take Raxone can be found in the treatment protocol document provided by the MHRA on Raxone.
In a series of online documents the MHRA has outlined in further details why Raxone was given a positive scientific opinion under the EAMS Programme and provides further detail on how Raxone should be taken by patients and administered by clinicians.
As always, the best place to hear up to the minute news is the Action Duchenne International Conference. Get tickets.


 Action Duchenne are Delighted to Announce Our New Campaigns Officer: Kathy
Action Duchenne are Delighted to Announce Our New Campaigns Officer: Kathy