Promising microdystrophin pre-clinical work, moving towards a potential therapy has shown to restore muscle function in the canine model (dogs). Golden Labradors are naturally affected by Duchenne muscular dystrophy so researchers chose them for mammalian trials, ahead of tests in humans. The new pre-clinical research carried out by researchers at the Royal Holloway in London and French scientists, showed that repairing the defective gene vastly improved the ability of dogs to run, walk and jump. The 12 dogs, who were not expected to live longer than six months, were treated as puppies and are still alive two years after the trial. In some cases, the dogs’ motor skills were indistinguishable from animals without the condition.
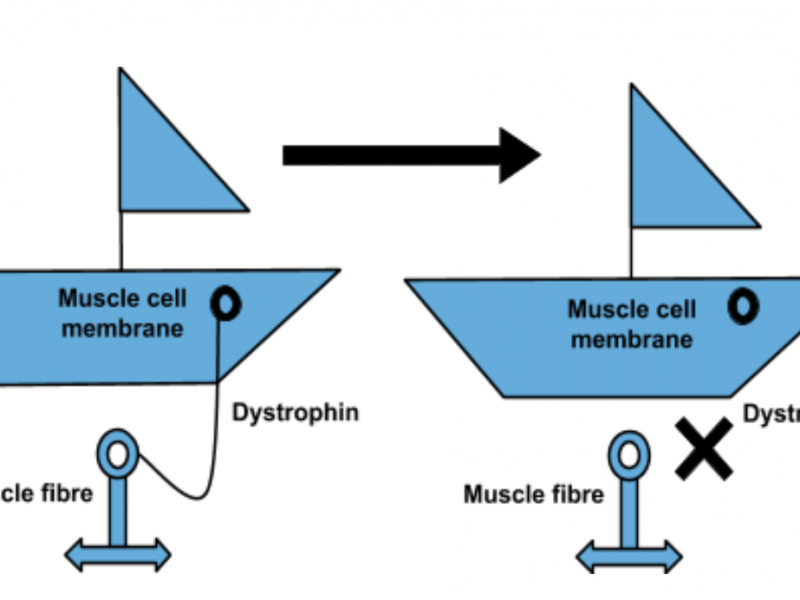
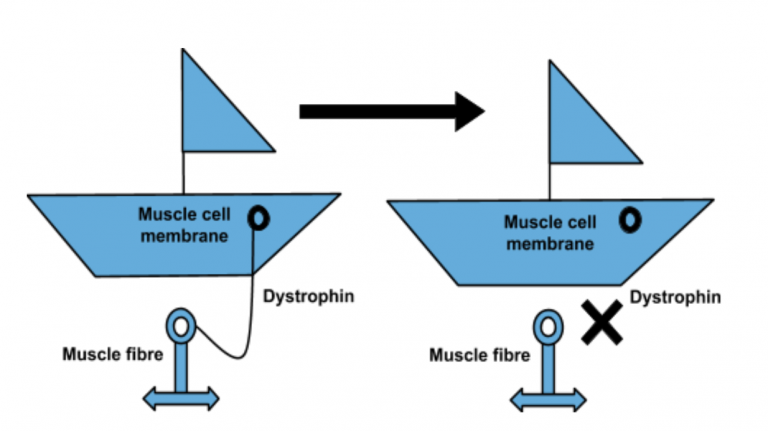
The potential microdystrophin therapy works by replacing a faulty gene with fully functioning DNA using a harmless virus, which ‘infects’ cells and alters their genetic code. Once repaired, the gene produces a protein which is essential for correct muscle functioning.
“In this seminal article in the high-impact journal Nature Communications, this study showed that micrdystrophin gene therapy significantly increased the amount of dystrophin protein in the dogs’ muscles, which led to an improvement in muscle function. Furthermore, the therapy did not induce a severe immune response in these investigations. This is one of the few studies which has been published which shows long-term data in the canine model, this is important evidence underpinning the potential of microdystrophin gene therapy and the reason which Action Duchenne has provided significant funds, alongside our partners AFM and MDUK towards the UNITE-DMD clinical trial project. “
Experts said the results offered ‘real hope’ for boys born with the condition.
Professor George Dickson, who led the trial at Royal Holloway, said: “This is tremendously exciting progress towards a gene therapy for Duchenne muscular dystrophy. The studies in dogs have been spectacular and exceeded our expectations.”
Click here for the full article published in Nature Communications.
What next?
In March 2017, Action Duchenne announced our funding of “UNITE-DMD”, the first gene therapy trials for Duchenne muscular dystrophy here in the UK.
From the very early days of Action Duchenne, we have supported research into gene therapy as a potential way of treating Duchenne muscular dystrophy. This area has seen significant progress in recent years, and now is the time to test its safety in people living with Duchenne, here in the UK.
This international collaboration will assess the safety of gene therapy in Duchenne, in a phase I/II clinical trial that will take place in both the UK and France. As these will be the first gene therapy trials for Duchenne in the UK, UNITE-DMD will be vital to test the safety of this technology.
Together with the principal funder Muscular Dystrophy UK, we are investing over £1.6m into UNITE-DMD. The French Muscular Dystrophy Association (AFM-Téléthon) is funding the French arm of the project.
As always the best place in the UK to get updates on all aspects of Duchenne is the annual Action Duchenne International Conference.
Contact our Director of Research, Neil Bennett with any questions neil@actionduchenne.org.

 Breaking news – first people enrolled in Raxone EAMS scheme in UK
Breaking news – first people enrolled in Raxone EAMS scheme in UK

