Action Duchenne is excited to share the news that the National Institute for Health and Care Excellence (NICE) has issued final draft guidance recommending vamorolone for the treatment of Duchenne muscular dystrophy (DMD).
This positive decision offers a step forward in the management of DMD, expanding the range of treatment options available to patients and their families, a currently urgent unmet need.
With the NICE’s approval, the NHS will now consider the recommendation and determine their next steps in this regulatory process to make vamorolone accessible to eligible patients under the NHS in England.
The Medicines and Healthcare products Regulatory Agency (MHRA) approved vamorolone for the treatment of Duchenne muscular dystrophy (DMD) in patients aged four years and older in January 2024. This was mirrored by The NICE’s final draft guidance. You should contact your neuromuscular clinician to discuss specific eligibility criteria.
The manufacturer (Santhera) has announced they intend to work closely with NHS England, NHS Wales and NHS Northern Ireland to ensure rapid patient access. Additionally, they are pursuing reimbursement via the Scottish Medicines Consortium (SMC) to ensure access for patients in Scotland
What is Vamorolone?
Vamorolone (often referred to as AGAMREE) is a novel ‘dissociative’ steroid, an anti-inflammatory agent similar to the currently available corticosteroids. Vamorolone binds to the same receptors as corticosteroids but alters the downstream activation pathways believed to be associated with a number of side effects reported with the long-term use of corticosteroids, particularly bone-weakening (osteoporosis). It is this reduced action that gives this novel steroid its dissociative label.
The focus on addressing side effects associated with long-term corticosteroid use is a welcome development. While vamorolone has the potential to reduce certain side effects, it is important to note that, like most medications, it may also have its own adverse effects.
According to the Patient Information Leaflet (PIL), available via the MHRA & NICE, the following side effects have been reported with Vamorolone (AGAMREE):
The following side effects have been reported with vamorolone at a very common frequency (may affect more than 1 in 10 people):
- More rounded, swollen aspect of the face (Cushingoid)
- Increase of body weight (weight increased)
- Increased appetite
- Irritability
- Vomiting
The following side effects have been reported at a common frequency (may affect up to 1 in 10 people):
- Belly pain (abdominal pain)
- Pain in the upper belly (abdominal pain upper)
- Diarrhoea
- Headache
The Journey So Far & Next Steps
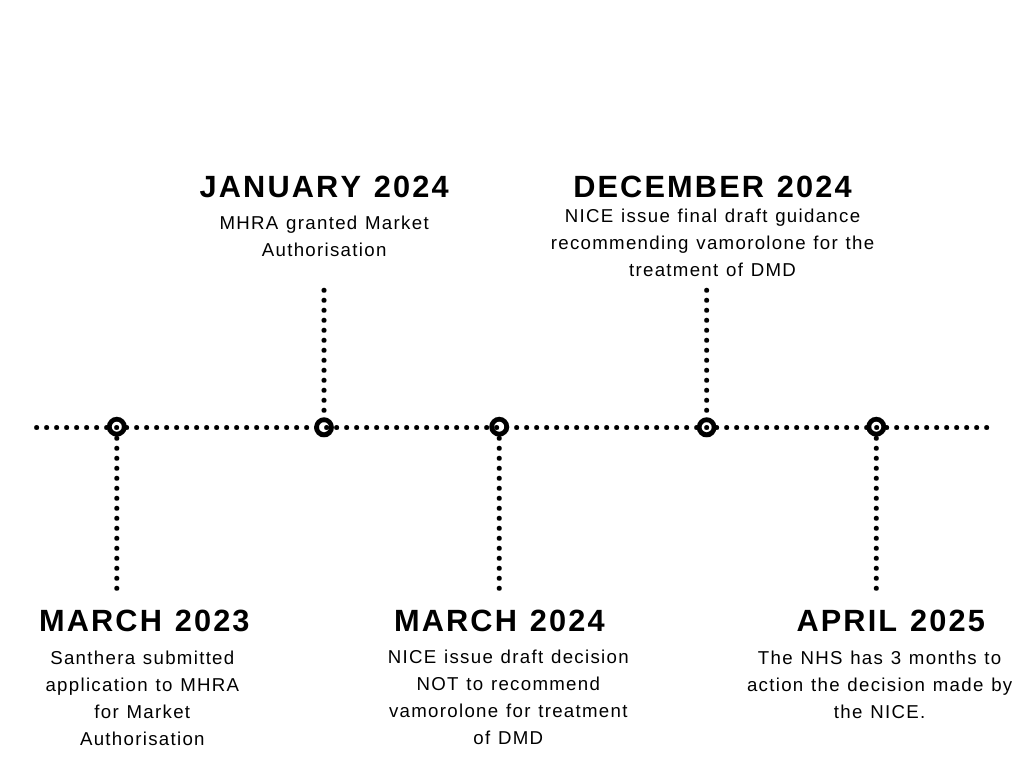
Firstly, the manufacturer (Santhera) submitted their clinical data where the safety and efficacy of the treatment was reviewed by the relevant regional bodies, such as the Medicines and Healthcare products Regulatory Agency (MHRA) in England.
The treatment was deemed to be safe and effective, and was granted market authorisation in January, 2024. Once the authorisation was obtained, regional bodies, such as the NICE in England, assessed the treatment based on cost effectiveness. The regulatory body then issues a recommendation on the treatment, which is then later considered by the NHS.
Vamorolone has just received its recommendation to treat DMD by the NICE, meaning NHS England will be reviewing the NICE decision, of which they have 3 months to implement. This includes updating guidelines, training healthcare professionals, and making the treatment available to eligible patients.
We await the answers to FAQs submitted to Santhera Pharmaceuticals (05/12/2024), the North Star Steering Committee (28/11/2024), and NICE (04/12/2024) and will provide an immediate update once this additional information is received. These FAQs have been compiled to help you navigate this announcement, so please stay tuned for updates.
We understand that this latest development will bring questions from the community, and we at Action Duchenne want to ensure families feel both fully informed and supported. If you have any questions or need support, please reach out to us at info@actionduchenne.org.




 REGENXBIO Announces Pivotal Phase of AFFINITY DUCHENNE Clinical Trial for RGX-202
REGENXBIO Announces Pivotal Phase of AFFINITY DUCHENNE Clinical Trial for RGX-202 
